Introduction
The Asia-Pacific endotoxin and pyrogen testing market is rapidly evolving with the increasing demand for safety and quality control in pharmaceuticals, biologics, and medical devices. Endotoxins, toxic substances released by Gram-negative bacteria, and pyrogens, fever-inducing agents, can cause serious adverse effects if present in injectable or implantable products. To comply with global regulatory frameworks, companies across the Asia-Pacific region are expanding their quality assurance capabilities through endotoxin and pyrogen testing.
The market is growing as countries in the region strengthen healthcare infrastructure, increase drug and vaccine manufacturing, and implement rigorous regulatory compliance standards. Asia-Pacific has become a global hub for clinical research, outsourcing, and pharmaceutical production, driving strong demand for high-sensitivity, reliable testing methods.
The Evolution
Endotoxin and pyrogen testing methods have evolved significantly from traditional animal-based approaches to modern in vitro techniques. The rabbit pyrogen test (RPT) was once the primary method to detect pyrogens in pharmaceutical products. However, ethical concerns and the need for more sensitive, scalable testing methods led to the adoption of the Limulus Amebocyte Lysate (LAL) assay. Derived from the blood of horseshoe crabs, the LAL test detects bacterial endotoxins with high sensitivity.
As environmental and sustainability concerns grew, particularly about the declining horseshoe crab population, alternative methods such as Recombinant Factor C (rFC) assays were developed. These do not require animal-derived materials and offer equivalent sensitivity to traditional LAL tests. Additionally, the Monocyte Activation Test (MAT) was introduced to detect both endotoxin and non-endotoxin pyrogens using human immune cells.
Countries like Japan, China, and South Korea have adopted modern testing techniques aligned with international pharmacopoeias. Emerging economies including India, Indonesia, and Vietnam are enhancing their compliance with global good manufacturing practices (GMP), further accelerating the evolution of testing protocols.
Source: https://www.databridgemarketresearch.com/reports/asia-pacific-endotoxin-and-pyrogen-testing-market
Market Trends
Biopharmaceutical growth is a major trend influencing the endotoxin and pyrogen testing market. The Asia-Pacific region is experiencing rapid growth in biologics and biosimilar production, particularly in China, India, South Korea, and Japan. These products require rigorous endotoxin testing at every stage of production.
Demand for rapid and automated testing solutions is rising. Companies are deploying systems that offer real-time results, high throughput, and minimized human error. This includes automated LAL readers and microfluidic-based testing platforms.
Sustainability is influencing technology adoption. Recombinant assays like rFC are gaining traction as companies adopt animal-free testing methods. Regulatory agencies in the region are beginning to recognize and approve these methods, which is encouraging wider implementation.
The COVID-19 pandemic highlighted the importance of vaccine safety and spurred investments in quality assurance. Vaccine manufacturers adopted rapid endotoxin testing methods to meet accelerated timelines. The long-term impact is a shift toward integrating advanced testing tools as part of standard manufacturing protocols.
Contract research organizations (CROs) and contract manufacturing organizations (CMOs) are expanding their service portfolios to include endotoxin and pyrogen testing. These players support small to mid-size pharmaceutical firms that may lack in-house capabilities.
Growing regulatory enforcement across Asia-Pacific is also shaping the market. Regulatory bodies are aligning more closely with US FDA, EMA, and ICH standards, compelling companies to improve their sterility assurance programs.
Challenges
The high cost of advanced testing methods such as recombinant assays and automated systems is a barrier for small and mid-sized manufacturers. Transitioning from traditional tests to new platforms requires capital investment and training.
Lack of harmonized regulations across Asia-Pacific countries creates compliance complexities. Manufacturers operating across borders must navigate varying standards, which can delay product approvals and increase operational costs.
Limited awareness and technical expertise in some emerging economies restrict the adoption of new testing methods. Laboratories may lack trained personnel or infrastructure to perform sophisticated assays such as MAT.
Conservation issues regarding the use of horseshoe crabs in LAL testing persist. Despite sustainable harvesting practices in some regions, environmental groups and regulatory stakeholders continue to push for alternatives.
Some biologic formulations interfere with LAL assays, necessitating validation and additional sample preparation. This increases the cost and complexity of testing.
Infrastructure gaps in rural or underdeveloped areas limit market expansion. Without proper cleanroom environments and laboratory setups, implementation of endotoxin and pyrogen testing becomes unfeasible.
Market Scope
By Test Type
-
LAL Test
-
Gel Clot Assay
-
Turbidimetric Assay
-
Chromogenic Assay
-
-
Recombinant Factor C (rFC) Assay
-
Monocyte Activation Test (MAT)
-
Rabbit Pyrogen Test (RPT)
By Application
-
Pharmaceuticals
-
Biologics and Biosimilars
-
Vaccines
-
Dialysis Fluids
-
Medical Devices
-
Injectable Drugs
-
Sterile Implants
By End User
-
Pharmaceutical Companies
-
Biotechnology Firms
-
Medical Device Manufacturers
-
CROs and CMOs
-
Academic Research Institutions
-
Hospital Labs
-
Regulatory Laboratories
By Country
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Singapore
-
Thailand
-
Malaysia
-
Vietnam
-
Indonesia
-
Rest of Asia-Pacific
Market Size
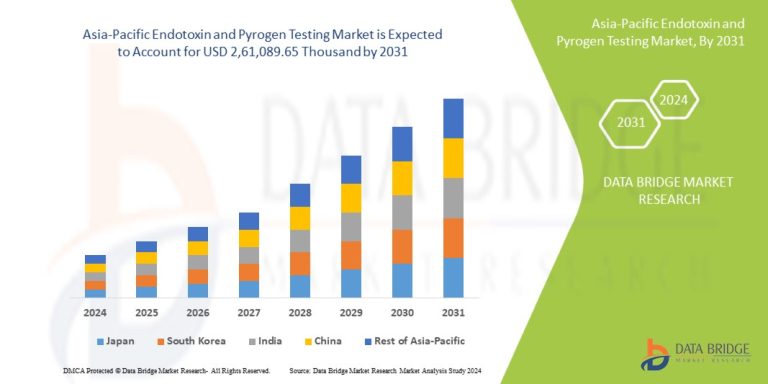
The Asia-Pacific endotoxin and pyrogen testing market was valued at USD 290 million in 2024 and is projected to reach USD 780 million by 2030, growing at a CAGR of 18.1% during the forecast period.
China is the largest market in the region, driven by its massive pharmaceutical industry, expanding vaccine production, and government initiatives to improve product safety. Japan and South Korea have mature markets with stringent regulations and high adoption of automated testing systems. India’s market is growing rapidly as the country increases biopharmaceutical exports and enhances GMP compliance.
The largest demand segment is pharmaceuticals, followed closely by medical devices and biologics. Vaccine applications have surged post-pandemic. Diagnostic labs and CROs represent the fastest-growing end user segment due to increased outsourcing.
Factors Driving Growth
Expansion of Biologics and Biosimilars
Asia-Pacific is emerging as a key manufacturing hub for biologics and biosimilars. These products require precise and consistent endotoxin testing throughout the development cycle. This creates a large and sustained demand for both in-process and final product testing.
Vaccine Manufacturing Growth
COVID-19 vaccination programs and investments in local vaccine production have dramatically increased testing needs. Countries are building new vaccine plants, all of which need robust quality control mechanisms, including pyrogen detection.
Increasing Contract Research and Manufacturing Services
CROs and CMOs in Asia-Pacific are growing, driven by outsourcing from global pharma companies. These organizations are expanding their capabilities, including endotoxin and pyrogen testing, to offer end-to-end service packages.
Regulatory Pressures
Regulators in the region are enforcing stricter quality standards. Countries like Japan, Singapore, and South Korea require validated endotoxin testing for approval of drugs and devices. China’s NMPA and India’s CDSCO are also aligning with global standards, pushing companies to upgrade testing methods.
Adoption of Automated and Recombinant Methods
The market is benefiting from the increased adoption of rFC-based tests and automated LAL systems. These provide high-throughput, real-time data and improved reproducibility while reducing animal testing.
Demand for Rapid and Reliable Testing
With more injectable and implantable products entering the market, manufacturers are adopting rapid endotoxin test kits and real-time monitoring systems to accelerate product release timelines.
Focus on Patient Safety
Hospitals and dialysis centers in Asia-Pacific are increasingly procuring devices and injectables with verified low pyrogen levels. This patient-centric demand enhances the role of quality assurance labs and testing facilities.
Global Supply Chain Integration
Asia-Pacific manufacturers are integrating into global pharmaceutical supply chains. To export to regulated markets like the US and EU, compliance with endotoxin testing standards is mandatory. This drives investment in sophisticated testing infrastructure.